Hello!
The other change that will occur in the bottle is that A. The air pressure will increase.
Why?
If volume changes in a closed container, while temeprature remains constant, the phenomenon is described by Boyle's Law:
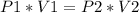
Let's give some arbitrary values to these variables:
P1= 1 atm
V1= 2 L
P2= ?
V2= 1 L
Calculating the final pressure (P2):
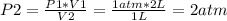
So, the final pressure is higher than the initial pressure (P2>P1). That means that Pressure and Volume are inversely proportional.
Have a nice day!