Answer:- Iron block final temperature is 64 degree C and copper block final temperature is 76 degree C.
Solution:- These problems are based on the formula:
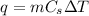
where, q is the heat energy, m is mass,
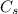 is specific heat and
is specific heat and
 is the change in temperature.
is the change in temperature.
mass of iron is given as 1.1 kg. We need to convert it to grams:
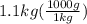 = 1100 g
= 1100 g
q is 13 kJ, let's convert it to J:
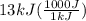 = 13000 J
= 13000 J
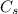 of iron is
of iron is
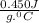
Let's calculate change in temperature for iron.
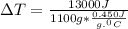
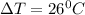
Since,
 = final temperature - initial temperature
= final temperature - initial temperature
26 = final temperature - 38
final temperature = 26 + 38 = 64 degree C
So, The final temperature of iron block would be 64 degree C.
Let's do the same calculations for copper block, it's mass is 890 g and specific heat is
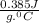 .
.
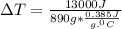
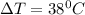
Now, calculations for final temperature:
 = final temperature - initial temperature
= final temperature - initial temperature
38 = final temperature - 38
final temperature = 38 + 38 = 76 degree C
So, the final temperature of copper block is 76 degree C.