Here we have to get the correct molecular formula of the compound.
The molecular formula of the compound is C₆H₆O₆ i.e. option C.
Let assume, the empirical formula of the compound is
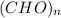 .
.
The given molar mass of the compound is 176.124 g/mole.
The percent of carbon in the compound is
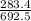 ×100 = 40.924.
×100 = 40.924.
The percent of hydrogen in the compound is
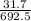 ×100 = 4.577.
×100 = 4.577.
The percent of oxygen in the compound is
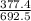 ×100 = 54.498.
×100 = 54.498.
Now the ratio of the atomic number in the compound for carbon is
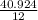 = 3.410
= 3.410
Now the ratio of the atomic number in the compound for hydrogen is
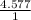 = 4.577
= 4.577
Now the ratio of the atomic number in the compound for oxygen is
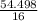 = 3.406
= 3.406
So, the C, H and O lowest ratio is
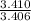 = 1,
= 1,
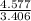 = 1 and
= 1 and
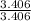 = 1
= 1
Thus the empirical formula of the compound is
![[CH_(1)O] _(n)](https://img.qammunity.org/2019/formulas/chemistry/high-school/gya9u61hoh6d28h0l7cmjzk0iu3h5zazry.png) (where n = integer.
(where n = integer.
12n + 1n + 16n = 176.24
29n = 176.24
n = 6 (approx)
Thus the molecular formula of the compound is C₆H₆O₆.