Answer: pOH of the given 0.135M solution of Pyridine is 9.137
Step-by-step explanation: Pyridine is a weak base and during its hydrolysis, pyridine forms pyridinium ion and releases hydroxide ions. Reaction follows:
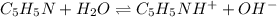
at
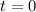 0.135M 0 0
0.135M 0 0
at
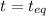 0.135-x x x
0.135-x x x
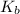 can be written as:
can be written as:
![K_b=([C_5H_5NH^+][OH^-])/([C_5H_5N])](https://img.qammunity.org/2019/formulas/chemistry/college/7lgzq47nztcb80alko1k1uljzw6jas1c1w.png)
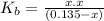
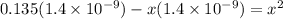
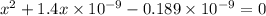
On solving the quadratic equation, we get
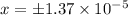
Negative value is neglected, as it is concentration and concentration cannot be in negative.
Therefore,
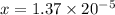
![x=[OH^-]=1.37* 10^(-5)](https://img.qammunity.org/2019/formulas/chemistry/college/50h18sa5arahppf0la5ev8duq74dl35d0v.png)
pOH can be calculated as:
![pOH=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bb6nd3dwmelw6hrf97fgv6xj1ptgdgg61f.png)
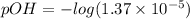
pOH = 4.863
pH can be calculated by:
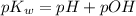
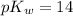
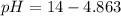
pH = 9.137