Answer: The correct option is B.
Explanation: All the given reactions are a type of single displacement reactions.
Single displacement reactions are defined as the reactions in which a more reactive metal displaces a less reactive metal in a chemical reaction.
General equation for these reactions is:
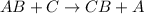
For the given reactions, we will use the reactivity series. The metal which lies above in the reactivity series will easily displace the metal which lies below in the reactivity series.
Option A: For this reaction, zinc lies below aluminium in the reactivity series. Hence, zinc will not be able to displace aluminium in the chemical reaction.
Option B: For this reaction, Sodium is more reactive than calcium as it lies above in the reactivity series. Hence, sodium will easily displace calcium in the given chemical reaction.
Reaction follows:
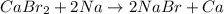
Option C: For this reaction, hydrogen lies below magnesium in the reactivity series. Hence, hydrogen will not be able to displace magnesium in the chemical reaction.
Option D: For this reaction, Calcium lies below barium in the reactivity series. Hence, calcium will not be able to displace barium in the chemical reaction.
Option E: For this reaction, barium lies below lithium in the reactivity series. Hence, barium will not be able to displace lithium in the chemical reaction.
Reactivity series is attached below.