Answer:-
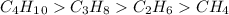
Explanations:- Alkanes are non polar molecules as these only have carbons and hydrogens. Electron negativity difference of C and H is very low and it makes them non polar. These have weaker London dispersion forces.
The forces of attraction becomes stronger in alkanes as the number of carbon increases because the surface area as well as molecular weight of the alkanes increases with an increase in number of carbons.
Butane has four carbons, propane has three carbons, ethane has two and methane has only one carbon, So, the strongest to weakest order of inter molecular forces is butane > propane > ethane > methane .