We have that the amount of water left in the container is
m'=0.4kg
From the question we are told
If you add 700 kJ of heat to 700 g of water originally at 70.0°C
The latent heat of vaporization of water is 22.6 x 105 J/kg
Generally the equation for 100C Heat is mathematically given as
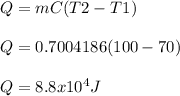
Generally the equation for the evaporation water Heat is mathematically given as
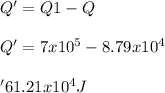
Hence with
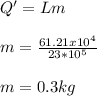
Therefore
The total Remaining within the contain will be
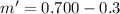
m'=0.4kg
Therefore
The amount of water left in the container is
m'=0.4kg
For more information on this visit