Answer: 2 moles of
 will be formed.
will be formed.
Explanation: Reaction in the given question is :

Number of moles of
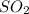 = 1 mole
= 1 mole
Number of moles of
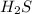 = 3 moles
= 3 moles
Here, the excess reagent is
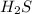 because its number of moles are more and
because its number of moles are more and
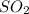 is the limiting reagent as its number of moles are less.
is the limiting reagent as its number of moles are less.
So, the amount of
 produced will depend on the the amount of
produced will depend on the the amount of
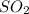 used.
used.
By stoichiometry, one molecule of
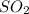 produces 2 molecules of
produces 2 molecules of
 .
.
Therefore, 1 mole of
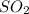 will produce 2 moles of
will produce 2 moles of
 .
.