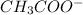Answer: The anion in the given formula is
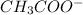
Step-by-step explanation:
Anion is the ion having negative charge. This is formed when an atom gains electrons.
The given compound is formed by the combination of lead ion and acetate ion.
Lead is the 82th element of the periodic table. The electronic confguration for this element is
![[Xe]4f^(14)5d^(10)6s^26p^2](https://img.qammunity.org/2019/formulas/chemistry/high-school/6fwba5g0q2uxq25dk289lyt72xnnoi6qql.png)
This element will loose 2 electrons and thus will form
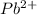 ion
ion
Acetate ion is the polyatomic ion having chemical formula of
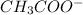 . This ion carries a charge of -1 unit.
. This ion carries a charge of -1 unit.
By using criss-cross method, the oxidation state of the ions are exchanged and it forms the subscript of the other ions. This results in the formation of a neutral compound.
Hence, the anion in the given formula is