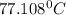Answer : The initial temperature of water (in
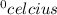 ) =
) =
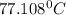
Solution : Given,
Mass of gold = 15.23 g
Mass of water = 28 g
Initial temperature of gold =
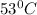
Final temperature of gold =
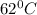
Final temperature of water =
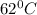
Heat capacity of gold =

Heat capacity of water =
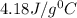
The formula used for calorimetry is,
q = m × c × ΔT
where,
q = heat required
m = mass of an element
c = heat capacity
ΔT = change in temperature
In the calorimetry, the energy as heat lost by the system is equal to the gained by the surroundings.
Now the above formula converted and we get
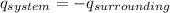
![m_(system)* c_(system)* (T_(final)-T_(initial))_(system)= -[m_(surrounding)* c_(surrounding)* (T_(final)-T_(initial))_(surrounding)]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/92n6ynvd6ep642fbfth0c03amw26eq38cp.png)
![m_(gold)* c_(gold)* (T_(final)-T_(initial))_(gold)= -[m_(water)* c_(water)* (T_(final)-T_(initial))_(water)]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/wrka8no3fhlvgq4xdmb26qgp8ieqg9ks66.png)
Now put all the given values in this formula, we get
![15.23g* 12.9J/g^(0)C * (62^(0)C-53^(0)C )= -[28g* 4.18J/g^(0)C * (62^(0)C-T_{\text{ initial of water}})]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/gx4rw2zhela6nfz80dbc4n744p1pduyxkp.png)
By rearranging the terms, we get

Thus the initial temperature of water =