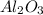Answer: 28.144 g of
![Al_2O_3[tex] will be produced.</strong></p><p><strong>Explanation:</strong> We are given that Al reacts with [tex]Fe_2O_3](https://img.qammunity.org/2019/formulas/chemistry/middle-school/wanae9e58zxvxl3xzhiksds4v2w11krrr3.png) , then the balanced chemical reaction is:
, then the balanced chemical reaction is:
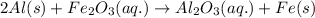
It is given that
 is present in excess, so it is an excess reagent and Al is the limiting reagent.
is present in excess, so it is an excess reagent and Al is the limiting reagent.
Now, 2 moles of Al produces 1 mole of
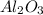 , which means that (2×27g) = 54g of Al produces 102g of
, which means that (2×27g) = 54g of Al produces 102g of
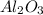 .
.
Therefore, 14.9g of Al will produce =
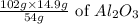
= 28.144g of