Answer: The correct option is D.
Step-by-step explanation: We are given two gas molecules with different masses. The collision between them is elastic, hence the Total Kinetic energy of the system is conserved.
Initial Kinetic Energy:
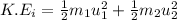
Final Kinetic Energy:
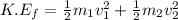
where,
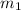 = mass of first molecule
= mass of first molecule
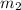 = mass of second molecule
= mass of second molecule
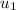 = initial velocity of first molecule
= initial velocity of first molecule
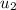 = initial velocity of second molecule
= initial velocity of second molecule
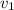 = final velocity of first molecule
= final velocity of first molecule
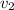 = final velocity of second molecule
= final velocity of second molecule
Elastic Collision:
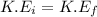
That is,
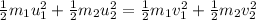
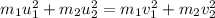 .....(1)
.....(1)
We are given in the question that the final velocity of first molecule is decreased by 1/4, which means:
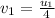
Putting this value in equation 1 , we get
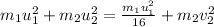
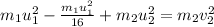
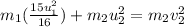
taking
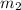 on other side, we get
on other side, we get
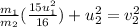
From the above relation, it is visible that the velocity change of the second molecule depends on both mass and velocity of the first molecule.