20 minutes.
The sample would lose one half the quantity of francium in each half-life.
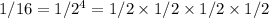
Thus a mass decrease by a factor of 16 would correspond to a period of four half-lives. It took 80 minutes for the sample to lose all these francium, therefore
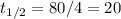 minutes.
minutes.