Answer: The solution of
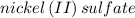 is green in color but a solution of sodium chloride is colorless that is why
is green in color but a solution of sodium chloride is colorless that is why
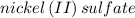 represent a suitable solution.
represent a suitable solution.
Step-by-step explanation:
The Beer-Lambert law represents the linear relationship between concentration and absorbance of an absorbing species according to the formula as follows.
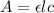
where A = absorbance
 = molar absorptivity
= molar absorptivity
l = length
c = concentration
It is known that
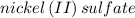 solution is green in color whereas sodium chloride solution is colorless. As a function of concentration, the solution of sodium chloride do not vary in absorbance.
solution is green in color whereas sodium chloride solution is colorless. As a function of concentration, the solution of sodium chloride do not vary in absorbance.
Therefore, it is concluded that solution of
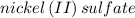 is green in color but a solution of sodium chloride is colorless that is why
is green in color but a solution of sodium chloride is colorless that is why
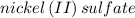 represent a suitable solution.
represent a suitable solution.