The actual amount of substance doesn’t matter for this problem, so assume an easy mass of compound using the percentages: 82.63 g C and 17.37 g H. There is also no need to worry about significant figures in an empirical formula problem.
Find the number of moles of each element:
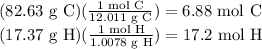
Find the ratio of the elements, and simplify until there are whole numbers that you can put into the empirical formula.
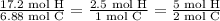
There are 5 mol H for every 2 mol C, so the empirical formula should be
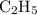 .
.