Answer :
a) The concentration of
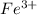 at equilibrium is 0.08 m.
at equilibrium is 0.08 m.
b) The concentration of
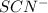 at equilibrium is 0.26 m.
at equilibrium is 0.26 m.
Solution :
The equilibrium reaction is,
![Fe^(3+)+SCN^-\rightleftharpoons [FeSCN]^(2+)](https://img.qammunity.org/2019/formulas/chemistry/high-school/p4ikzvaw6h7j17ormsdunk6vjsrrtcrvwn.png)
Initial concentration 0.230 0.410 0
At equilibrium (0.230 - x) (0.410 - x) x
Given x = 0.150
Therefore,
The concentration of
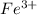 = 0.230 - x = 0.230 - 0.150 = 0.08
= 0.230 - x = 0.230 - 0.150 = 0.08
The concentration of
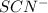 = 0.410 - x = 0.410 - 0.150 = 0.26
= 0.410 - x = 0.410 - 0.150 = 0.26
Thus, the concentration of
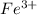 at equilibrium is 0.08 m.
at equilibrium is 0.08 m.
The concentration of
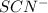 at equilibrium is 0.26 m.
at equilibrium is 0.26 m.