Answer : The pressure of
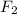 is 0.975 atm.
is 0.975 atm.
Solution : Given,
Mole fraction of
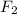 = 0.25
= 0.25
Mole fraction of
 = 0.65
= 0.65
Mole fraction of
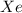 = 0.10
= 0.10
Total pressure of mixture = 3.9 atm
According to the Dalton's law of partial pressure, which tells that the partial pressure of a gas is directly proportional to the mole fraction of a gas.
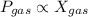

where,
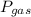 = partial pressure of a gas
= partial pressure of a gas
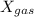 = mole fraction of a gas
= mole fraction of a gas
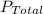 = total pressure of gas
= total pressure of gas
Now we have to calculate the partial pressure of
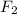 gas.
gas.
Put all the given values in above formula, we get
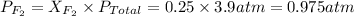
The partial pressure of
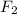 is 0.975 atm.
is 0.975 atm.