Answer: The given compound (water) is a pure substance
Step-by-step explanation:
A pure substance is defined as the substance which is formed by same or different type of atoms which are chemically combined in a fixed ratio by mass.
Compounds and elements are considered as pure substances
A mixture is defined as the substance which is made by mixing two or more substances in any ratio. They can be separated by physical means.
Water
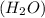 is formed by the combination of hydrogen and oxygen elements in z fixed ratio of 1 : 8 by mass. Therefore, it is considered as a compound.
is formed by the combination of hydrogen and oxygen elements in z fixed ratio of 1 : 8 by mass. Therefore, it is considered as a compound.
Hence, the given compound (water) is a pure substance