Answer:
Released energy,
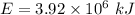
Step-by-step explanation:
It is given that,
Loss in mass in a nuclear reaction,
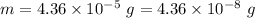
The relation between the mass and energy is given by Einstein mass energy equivalence equation :
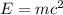
c is the speed of light
So,
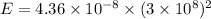
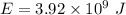
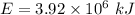
The energy released in a nuclear reaction is
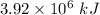 . Hence, the correct option is (B)
. Hence, the correct option is (B)