Answer : Electron P has greater energy difference than the Electron N.
Explanation :
Wavelength range of violet light = 400 - 500 nm
Wavelength range of orange light = 600 - 700 nm
The Planck's equation is,
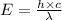
where,
E = energy of light
c = speed of light
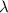 = wavelength of light
= wavelength of light
According to the Planck's equation, wavelength and energy follow inverse relation. As the wavelength increases, energy decreases.
From the given spectrum, the wavelength of violet light is less. We conclude that When electron P gives violet light on transition it means that energy difference between the energy level was high.
From the given spectrum, the wavelength of orange light is more. We conclude that When electron N gives orange light on transition it means that energy difference between the energy level was low.
So, Electron P which gives violet light on transition has greater energy difference than the Electron N.