Answer:- Molecular formula of the compound is
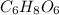 .
.
Solution:- Grams of C, H and O are given from which the moles are calculated. Moles of each element are divided by the least one of them to get the mole ratio. If it's not a whole number ratio then we multiply the ratios by an smallest integer like 2,3,4------etc to get the whole number ratio that gives the empirical formula.
Molar mass is divided by the empirical formula mass to know how many empirical formula units are actually present in the compound. The calculations for all the steps are shown below:
calculations for moles:
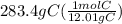
= 23.6 mol C
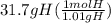
= 31.4 mol H
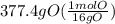
= 23.6 mol O
Calculations for mol ratio:
In the above moles of C, H and O, the least number is 23.6. So, let's divide each of them by 23.6.
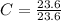 = 1
= 1
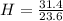 = 1.33
= 1.33
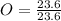 = 1
= 1
1.33 is not a whole number so we need to multiply all of these by 3 that gives almost whole number ratio.
C = 3(1) = 3
H = 3(1.33) = 3.99 = 4
O = 3(1) = 3
So, the empirical formula of the compound is
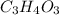 .
.
Empirical formula mass = 3(12.01)+4(1.01)+3(16)
= 36.03+4.04+48
= 88.07
Number of empirical formula units =
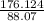 = 2
= 2
So, molecular formula of the compound contains two empirical formula units and the molecular formula is
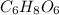 .
.