It takes 33.4 s for the concentration of A to fall to one-fourth of its original value.
A half-life is the time it takes for the concentration to fall to half its original value.
Assume the initial concentration is 1.00 mol/L. Then,
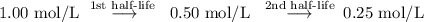
The concentration drops to one-fourth of its initial value in two half-lives.
∴ Time = 2 × 16.7 s = 33.4 s