Answer: The correct answer is Option D.
Step-by-step explanation:
Weak acid is defined as the acid which does not completely dissociates into its ion when dissolved in water.
For the given options:
Option A:
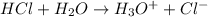
HCl is a strong acid because it completely looses its proton when dissolved in water.
Option B:
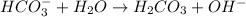
Hydrogen carbonate ion is a base because it is easily accepting a proton from water.
Option C:
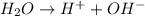
The above equation represents the ionization of water.
Option D:
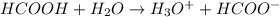
Formic acid is considered as a weak acid because it does not completely dissociates into its ions when dissolved in water.
From the above information, the correct answer is Option D.