Answer : The correct option is, three, dumbbell.
Explanation :
As we know that there are many sub-levels which are s, p, d, f, g and so on. The sub-level are oriented in three directions along the x, y and z axis.
The 's' sub-level has one orbital that are spherically shaped.
The 'p' sub-level has three orbitals
 that are dumbbell shaped.
that are dumbbell shaped.
The 'd' sub-level has five orbitals
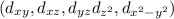 that are double-dumbbell shaped.
that are double-dumbbell shaped.
The 'f' sub-level has seven orbitals
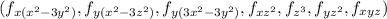 that are complex flower shaped.
that are complex flower shaped.
Hence, the 'p' sub-level has three orbitals that are dumbbell shaped.