Answer: saturated
Explanation:
Saturated hydrocarbons are defined as the hydrocarbons in which a single bond is present between carbon and carbon atoms. The general formula for these hydrocarbons is
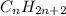 .
.
Unsaturated hydrocarbons are defined as the hydrocarbons which have double or triple covalent C-C bonds. They are known as alkenes and alkynes respectively. The general formula for these hydrocarbons is
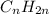 and
and
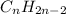 .
.
The given compound is
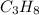 belongs to formula
belongs to formula
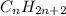 where n=3 and thus a saturated hydrocarbon.
where n=3 and thus a saturated hydrocarbon.