Answer:- Ca = +2, S = +6 and O = -2
Solution:- There are certain rules for oxidation numbers. As per the rule, oxidation number of alkaline earth metals in their compounds is +2.
Oxidation number of oxygen in it's compounds is -2(except peroxides) and the sum of oxidation numbers of all the elements of a neutral compound is zero.
Since, Ca is +2 and O is -2, the oxidation number of S could easily be calculated for the given compound as:
Let's say the oxidation number of S in
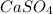 is
is
 . Let's make the algebraic equation and solve it.
. Let's make the algebraic equation and solve it.
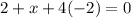
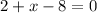
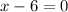
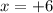
Hence. the oxidation number of Ca is +2, O is -2 and S is +6.