Answer:- D.
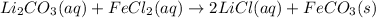
Explanations:- In chemical equations, (aq) stands for aqueous, (l) stands for liquid, (g) stands for gas and (s) stands for solid. In double replacement reactions, (aq) means soluble and (s) means insoluble that is precipitate.
Looking at all the equations, it is only the last equation that has a solid product and so this is the only precipitation reaction. A precipitate for Iron(II)carbonate is formed in it.