Answer:-
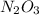 has 2 atoms of nitrogen and 3 atoms of oxygen.
has 2 atoms of nitrogen and 3 atoms of oxygen.
 has 1 atom of calcium, 1 atom of carbon and 3 atoms of oxygen.
has 1 atom of calcium, 1 atom of carbon and 3 atoms of oxygen.
Explanations:- The numbers written as subscripts for the elements in formulas of the compounds represents the number of atoms of that particular element.
The first compound is
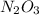 and in this the subscript of N is 2 and that of O is 3. It means it has 2 atoms of N and 3 atoms of O.
and in this the subscript of N is 2 and that of O is 3. It means it has 2 atoms of N and 3 atoms of O.
Second compound is
 and in this the subscript of Ca is not shown it means it is 1, similarly the subscript of C is 1 and subscript of O is 3. So, the compound has 1 atom of Ca, 1 atom of C and 3 atoms of O.
and in this the subscript of Ca is not shown it means it is 1, similarly the subscript of C is 1 and subscript of O is 3. So, the compound has 1 atom of Ca, 1 atom of C and 3 atoms of O.