Answer:
Mass of the gold bar is 90.71 gram.
Step-by-step explanation:
Given:
volume = 4.7
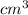
density = 19.3
To find:
mass = ?
Formula used:
density =
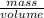
Solution:
density is given by,
density =
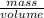
mass = volume × density
mass = 4.7 × 19.3
mass = 90.71 g
Mass of the gold bar is 90.71 gram.