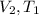Answer : The variables in the numerator (a) will be,
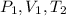 and the variables in the denominator (b) will be,
and the variables in the denominator (b) will be,
Explanation :
The combined gas law has arrived from the combination of the four laws:
1) Boyle's Law : It is defined as the pressure of the gas is inversely proportional to the volume of the gas at constant temperature and the number of moles.
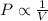
2) Charles' Law : It is defined as the volume of the gas is directly proportional to the temperature of the gas at constant pressure and number of moles.
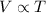
3) Gay-Lussac's Law : It is defined as the pressure of the gas is directly proportional to the temperature of the gas at constant volume and number of moles.
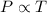
4) Avogadro's Law : It is defined as the volume of the gas is directly proportional to the number of moles of the gas at constant pressure and temperature.
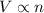
By combining these four laws, we get the combined gas law.
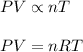
where, R = gas constant
An ideal gas at initial pressure
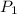 , initial volume
, initial volume
 and initial temperature
and initial temperature
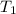 undergoes changes with the variables to final pressure
undergoes changes with the variables to final pressure
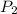 , final volume
, final volume
 and final temperature
and final temperature
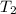 .
.
By rearranging the gas law, we get the final pressure.
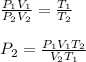
Therefore, the variables in the numerator (a) will be,
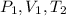 and the variables in the denominator (b) will be,
and the variables in the denominator (b) will be,