Answer: the lattice contains 1 magnesium ion for every 2 fluoride ions.
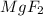 is an ionic compound. It contains positive ions of Magnesium and and negative ions of Fluorine. To balance the +2 positive charge of one magnesium ion, two (-1) negatively charged Fluorine ions are present. Hence, The correct interpretation of
is an ionic compound. It contains positive ions of Magnesium and and negative ions of Fluorine. To balance the +2 positive charge of one magnesium ion, two (-1) negatively charged Fluorine ions are present. Hence, The correct interpretation of
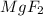 is that the lattice contains 1 Magnesium ion for every 2 Fluoride ions.
is that the lattice contains 1 Magnesium ion for every 2 Fluoride ions.