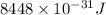Answer:
The wavelength, frequency and speed of a photon are related as:
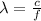
where,
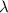 is the wavelength, c is the speed of light because all photons travels with speed of light, c in space, and f is the frequency.
is the wavelength, c is the speed of light because all photons travels with speed of light, c in space, and f is the frequency.
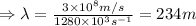
Hence, the wavelength of the radio photon is 234 m
Energy of this photon:
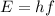
where, h is the Planck's constant.
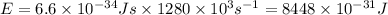
Hence, the energy of the radio photon is