Answer:- work = 319 J
Solution:- The volume of the gas decreases from 5.35 L to 1.55 L.
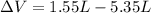 = -3.80 L
= -3.80 L
external pressure is given as 0.829 atm.
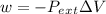
where w is representing work.
Let's plug in the values and calculate pressure-volume work:
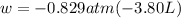
w = 3.15 atm.L
We need to convert atm.L to J.
1 atm.L = 101.325 J
So,
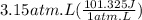
= 319 J
So, 319 J of work is involved in a chemical reaction. Positive work means the work is done on the system.