Answer:- 27.7 grams of
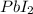 are produced.
are produced.
Solution:- The balanced equation is:
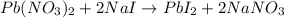
let's convert the grams of each reactant to moles and calculate the grams of the product and see which one gives least amount of the product. This least amount would be the answer as the least amount we get is from the limiting reactant.
Molar mass of
![Pb(NO_3)_2 = 207.2+2(14.01)+6(16) = 331.22 gram per mol</p><p>molar mass of NaI = 22.99+126.90 = 149.89 gram per mol</p><p>Molar mass of [tex]PbI_2](https://img.qammunity.org/2019/formulas/chemistry/middle-school/2or33ugt2rzmpzyjqpp5djjx4d7j18ml2j.png) = 207.2+2(126.90) = 461 gram per mol
= 207.2+2(126.90) = 461 gram per mol
let's do the calculations for the grams of the product for the given grams of each of the reactant:
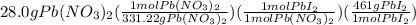
=
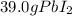
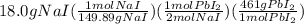
=
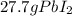
From above calculations, NaI gives least amount of
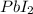 , so the answer is, 27.7 g of
, so the answer is, 27.7 g of
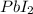 are produced.
are produced.