Answer:- 12.7%
Solution:- It says, ammonium phosphate is 60.9%. It means if we assume 100 g of the sample then there is 60.9 g ammonium phosphate in it.
Formula of ammonium phosphate is
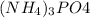 and it's molecular weight is 149 grams per mol. There is only one P in the formula and so the mass of P in 149 g of ammonium phosphate is 31 g(atomic mass of P).
and it's molecular weight is 149 grams per mol. There is only one P in the formula and so the mass of P in 149 g of ammonium phosphate is 31 g(atomic mass of P).
Now, we could use the proportions to find out the mass percentage of P in the sample.
31 g P is present in 149 g of ammonium phosphate, how many grams of P would be present in 60.9 g of ammonium phosphate. Let's set the proportions as:
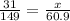
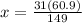
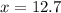
This 12.7 g of phosphorous is present in 100 g of the sample means there is 12.7% phosphorous in the sample.