Answer:
The molar mass of the gas is 70.83 g/mol.
Step-by-step explanation:
Mass of the gas m= 827 mg = 0.827 g
Molar mass of gas = M
Pressure of the gas = 975 mmHg = 1.28 atm
Temperature of the gas = 88^C= 361 K
Using idal gas equation:
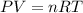
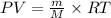
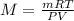
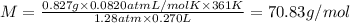
The molar mass of the gas is 70.83 g/mol.