Answer: Theoretical mass of sodium sulphate (
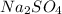 ) is 514.118 grams.
) is 514.118 grams.
Step-by-step explanation: For a given reaction,
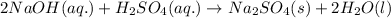
As NaOH is used in excess, therefore it is an excess reagent and
 is a limiting reagent as the quantity of the product will depend on it.
is a limiting reagent as the quantity of the product will depend on it.
We are given 355 grams of
 .
.
Molar mass of
 = 98.079 g/mol
= 98.079 g/mol
Molar mass of
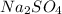 = 142.04 g/mol
= 142.04 g/mol
1 mole of
 is producing 1 mole of
is producing 1 mole of
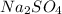 , so
, so
98.079 g/mol of
 will produce 142.04 g/mol of
will produce 142.04 g/mol of
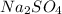
355 grams of
 will produce =
will produce =
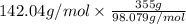 of
of
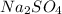
Mass of
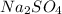 = 514.118 grams
= 514.118 grams