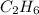Answer:- 3.
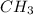 and
and
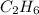
Explanations:- An empirical formula is the simplest whole number ratio of atoms of each element present in the molecule/compound.
For example, the molecular formula of benzene is
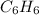 . The ratio of C to H in it is 6:6 that could be simplified to 1:1. So, an empirical formula of benzene is CH.
. The ratio of C to H in it is 6:6 that could be simplified to 1:1. So, an empirical formula of benzene is CH.
In the first pair, the ratio of C to H in first molecule is 2:4 that could be simplified to 1:2 and the empirical formula is
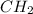 . In second molecule the ratio of C to H is 6:6 and it could be simplified to 1:1. and the empirical formula is CH. Empirical formulas are different for both the molecules of first pair and so it is not the right choice.
. In second molecule the ratio of C to H is 6:6 and it could be simplified to 1:1. and the empirical formula is CH. Empirical formulas are different for both the molecules of first pair and so it is not the right choice.
In second pair, C to H ratio in first molecule is 1:2, so the empirical formula is
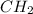 . The C to H ratio for second molecule is 1:4, so the empirical formula is
. The C to H ratio for second molecule is 1:4, so the empirical formula is
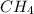 . Here also, the empirical formulas are not same and hence it is also not the right choice.
. Here also, the empirical formulas are not same and hence it is also not the right choice.
In third pair, C to H ratio in first molecule is 1:3, so the empirical formula is
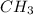 . In second molecule the C to H ratio is 2:6 and it is simplified to 1:3. So, the empirical formula for this one is also
. In second molecule the C to H ratio is 2:6 and it is simplified to 1:3. So, the empirical formula for this one is also
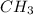 . Hence. this is the correct choice.
. Hence. this is the correct choice.
In fourth pair, first molecule empirical formula is CH. Second molecule has 2:4 that is 1:2 mole ratio of C to H and so its empirical formula is
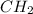 . As the empirical formulas are different, it is not the right choice.
. As the empirical formulas are different, it is not the right choice.
So, the only and only correct pair is the third one. 3.
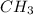 and
and