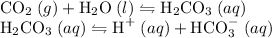C)
 hydrochloric acid and, in case that the question allows for more than one choices, D)
hydrochloric acid and, in case that the question allows for more than one choices, D)
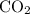 carbon dioxide as well.
carbon dioxide as well.
Methane molecules
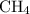 are nonpolar and barely dissolve in water.
are nonpolar and barely dissolve in water.
Sodium hydrocarbonate
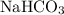 undergoes hydrolysis to release hydroxide ions
undergoes hydrolysis to release hydroxide ions
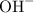 that can end up consuming
that can end up consuming
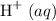 :
:
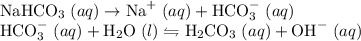
Hydrochloric acid, a strong acid, ionizes to produce protons
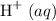 and chloride ions when dissolved in water:
and chloride ions when dissolved in water:
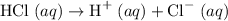
Carbon dioxide reacts with water to produce hydrocarbonic acid, a weak acid that slightly ionizes, also producing protons
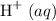 but to a significantly lesser extent than hydrochloric acid does.
but to a significantly lesser extent than hydrochloric acid does.