Answer:
Water is a simple compound.
Step-by-step explanation:
Water, from first glance, is a simple compound. This is because it is made up of two atoms - hydrogen and oxygen. This gives the formula:

In the simple compound, one atom of oxygen is bound to two hydrogen atoms like this:
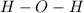
The bonding in water is hydrogen bonding. This is a bonding that takes place between the oxygen atom and a hydrogen atom of the next water molecule.
This makes the water molecule an interesting molecule.