Answer: Option (c) is the correct answer.
Step-by-step explanation:
Calcium is a group 2 element and it lies in period 4 and group 2.
Atomic number of calcium is 20 and its electronic distribution is 2, 8, 8, 2. That is, in ground state there are 20 electrons present in a calcium atom.
It's electronic configuration is as follows.
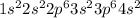
Thus, we can conclude that calcium's ground-state electron configuration is
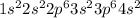 .
.