Answer:
1.A
2.D
3.D
Step-by-step explanation:
1.We have to fill correct answer in blank space given in the question.
Bohr's postulates
1.Electron revolve in certain stable orbits around the nucleus without radiating any energy .The stable orbits or energy levels are called stationary orbits and these orbits at a certain discrete distance from the nucleus.
2.The stationary orbits are attained at distances for which the angular momentum is integral multiple of plank;s constant
L=mvr=nh
Where n is called principle quantum number
3.Electrons gain or lose energy when they jump from one energy lever to other energy lever or when higher energy level to low energy level.
Hence, the mystery of periodic laws was solved when Bohr proposed his planetary atomic model of the atom ,providing an understanding of the electronic structure of the elements and the organization of electrons into shells,
Option A is true.
2.When am electron absorb energy then the electrons jump from low energy level to high energy level .When the electron return from higher energy level to low energy level then it emit colored flame.
When Christ put copper chloride into Bunsen burner flame then it began to emit a green color because when excited electron returns back to the ground state , then photon of light is emitted.
Hence, option D is true.
3.Boron-10 has abundance 19.8% and actual mass of 10.013 amu and boron-11 has an abundance of 80.2% and actual mass of 11.009.
We have to find the average atomic mass for all isotopes of boron
Average atomic mass of boron =
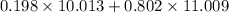
Average atomic mass of boron=10.811792=10.812 amu
Hence, option D is true.