Answer: Option (B) is the correct answer.
Step-by-step explanation:
Molar mass is defined as the sum of atomic mass of all the atoms present in a compound.
For example, atomic mass of barium (Ba) is 137.32 g/mol and atomic mass of a bromine atom is 79.90 g/mol.
Therefore, calculate molar mass of
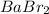 as follows.
as follows.
Molar mass = atomic mass of Ba + 2
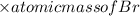
=
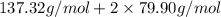
= 297.12 g/mol
Therefore, we can conclude that molar mass of
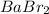 is 297.1 g/mol.
is 297.1 g/mol.