Answer
The answer is (C) as
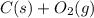 →
→

Step-by-step explanation
It is commonly known that oxygen has the importance in the combustion reaction and in the absence of this molecule, the no combustion reaction will proceed. SO, for the combustion reaction, it is important that oxygen must be present in the reactant side of the equation than on the product side. Another identity is also present in the combustion reaction as carbon dioxide and water will be present in the product side. In the present options, the oxygen is only present in equation (C) in the reaction side.
So, the right option is
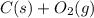 →
→
