Answer:
E = 50850[kJ]
Step-by-step explanation:
To boil the water we must use the latent heat of vaporization. Also we need to multiply this value by the mass of the water.
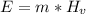
Where:
E = energy produced by heat [J]
m = mass = 225 [kg]
Hv = 226000 [J/kg]
![E = 225*226000\\E = 50850000 [J]\\E=50850[kJ]](https://img.qammunity.org/2022/formulas/physics/high-school/5bssw89ic3ixlan1tn5v7uofsl71zayv4v.png)