From the ideal gas equation,
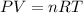
where n is number of moles, R is Universal gas constant, P is pressure, V is volume, and T is temperature of the gas.
The pressure and volume are inversely proportional to each other at constant temperature and number of moles.
Hence, on decreasing the pressure, the volume will increase.
As the hiker reaches a height of a mountain, the pressure would decrease which results in the reestablishment of equilibrium between gas molecules thus resulting in pushing of bag outwards.
Hence, the bag will expand as the hiker reaches the top of the mountain.