According to Charles' Law the volume of an ideal gas is directly proportional to its absolute temperature in Kelvin keeping the pressure constant.
V∝ T, P is constant
where V, T and P are volume, temperature and pressure
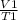 =
=
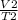
where V₁, T₁, V₂ and T₂ are initial volume, initial temperature, final volume and final temperature.