Answer:
The gold gains a total of 2.468 kilo-joules of energy.
Step-by-step explanation:
Total heat or energy gained by the gold is equal to heat applied and heat required to melt the gold completely.
Total energy = Q+ Q'
Heat of fusion of gold =
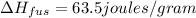
Mass of gold melted ,m= 12.5 g
Specific heat of gold ,c= 0.1291 joules/gram °C
Change in temperature = ΔT = 1064°C - 26°C = 1038 °C
Heat of applied to the gold = Q
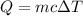
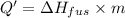
Total energy = Q+ Q'
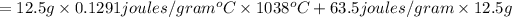
= 2,468.82 Joule= 2.468 kilo-Joule
The gold gains a total of 2.468 kilo-joules of energy.