Answer :
(D)
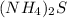 (C)
(C)
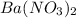 (B)
(B)
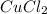
(A)
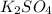 _
_
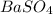 _
_
(B)
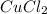
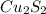 _
_
(C)
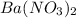 BaS
BaS
Explanation :
(1) Reaction for A + B
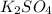 +
+
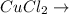 2KCl +
2KCl +
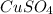
In this reaction, there is no precipitate formed as the products are souble.
(2) Reaction for A + C
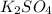 +
+
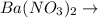
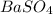 +
+
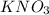
In this reaction,
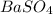 is formed as precipitate as it do not dissolve.
is formed as precipitate as it do not dissolve.
(3) Reaction for A + D
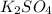 +
+
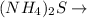
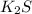 +
+
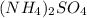
In this reaction, there is no precipitate formed as the products are souble.
(4) Reaction for B + D
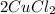 +
+
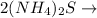
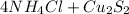
In this reaction,
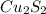 is formed as precipitate as it do not dissolve.
is formed as precipitate as it do not dissolve.
(5) Reaction for B + C
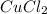 +
+
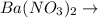
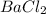 +
+
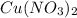
In this reaction, there is no precipitate formed as the products are souble.
(6) Reaction for C + D
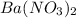 +
+
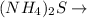 BaS +
BaS +
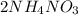
In this reaction, BaS is formed as precipitate as it do not dissolve.