Answer:- The given ion has 41 protons, 52 neutrons and 38 electrons.
Solution:- Atomic number for Nb is 41 and it's mass number is given as 93.
We know that, atomic number = number of protons
Since the atomic number is 41, it has 41 protons.
For a neutral atom, number of electrons = number of protons
Since the element has +3 charge, It must have lost three electrons to form the ion.
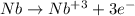
So, the number of electrons for the given ion = 41 - 3 = 38
number of neutrons = mass number - number of protons
number of neutrons = 93 - 41 = 52
So, the given ion has 41 protons, 52 neutrons and 38 electrons.